Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Hydrogen Towards a Sustainable Energy Future - The Indian Scenario
Authors: Jayant Jain, Aahil Khambhawala , Rashmi Kumar
DOI Link: https://doi.org/10.22214/ijraset.2023.54583
Certificate: View Certificate
Abstract
Hydrogen is considered an alternative fuel for automobiles due to its ability to power fuel cells in zero-emission vehicles (vehicles with no emissions of air pollutants), the possibility of domestic production, and the potential for high fuel cell efficiency all contribute to the interest in hydrogen as an alternative transportation fuel. This paper lays out the current scenario of R&D activity in India and look at the various governmental policies that facilitate this development across several program areas. The paper also discusses the current scenario of projects implemented by both the private and the public sector on large scale green hydrogen production and development and deployment of hydrogen vehicles.
Introduction
I. INTRODUCTION
90 Million Metric Tons of Hydrogen is produced every year globally through various sources, including 59% from Natural Gas, 19% from coal, 0.7% from fossil fuels, 0.6% from oil, and the remaining 21% from a by-product in other industrial processes. In India, 6 Million Metric Tons of Hydrogen are produced every year out of which 2.6 Million Metric Tons are utilized in refineries, 3.2 Million Metric Tons in the Chemical and Fertilizer industries, and the remaining for other purposes.

According to the US Energy Policy Act of 1992, hydrogen qualifies as an alternative fuel for automobiles[1]. The ability to power fuel cells in zero-emission vehicles (vehicles with no emissions of air pollutants), the possibility of domestic production, and the potential for high fuel cell efficiency all contribute to the interest in hydrogen as an alternative transportation fuel. A fuel cell might be two to three times more efficient than a gasoline-powered internal combustion engine. While hydrogen can also be used to power internal combustion engines, doing so produces nitrogen oxide emissions and is less effective than using it to power fuel cells. Light-duty hydrogen fuel cell vehicles are offered for leasing by several automakers.
Given that it emits practically nothing except water, it has the advantage of being a clean energy source and is used in numerous applications. The so-called new hydrogen economy is one of the possibilities for a sustainable energy supply because it can be created from any source of energy, with renewable energy being the most alluring.
Despite the growing interest in hydrogen as a fuel source, its primary use remains in the production of ammonia, metals, and electronics, with an annual global consumption of roughly 40 million tons. As a result, large-scale hydrogen generation is needed to meet this large-scale hydrogen consumption. Currently, pyrolysis and reformation of heavy crude, gasification and reformation of coal, and natural gas reforming are the technologies that are most commonly used to produce hydrogen.
II. METHODS OF HYDROGEN PRODUCTION
A. Grey Hydrogen Production Methods
The generation is using Natural Gas as a primary source of fuel and since natural gas is a cheaper alternative to fuel, this process is widely used in industries. Grey Hydrogen production is rarely supported by carbon capture (CCUS). In this process, the production of one tonne of hydrogen results in the production of 10 tons of carbon dioxide[2].
- Steam Methane Reforming (SMR)
One of the most popular and affordable technologies for producing hydrogen at the moment is steam reforming. The following is the network of reforming reactions for methanol and hydrocarbons used as feedstock[3]:
CmHn + mH2O (g) → mCO + (m + 0.5n) H2 Equation 1
CmHn + 2mH2O (g) → mCO2 + (2m + 0.5n) H2 Equation 2
CO + H2O (g) ↔ CO2 + H2 Equation 3
CH3OH + H2O (g) ↔ CO2 + 3H2 Equation 4
There are two steps to the entire process. The hydrocarbon raw material is mixed with steam and supplied into a tubular catalytic reactor in the first stage. Syngas (an H2/CO gas mixture) with a lower CO2 content is produced during this process. Oxygen or air is added to raise the reaction temperature as needed when a portion of the raw material (heated gas) inside the reactor is burned. The second stage involves feeding the cooled product gas into the CO catalytic converter, primarily using steam to convert carbon monoxide into carbon dioxide and hydrogen. To prevent the deactivation of the catalyst employed in the steam reforming catalytic process, a raw material devoid of compounds containing sulphur is required[3]. Two procedures are used in steam reforming: the standard process and the Pressure Swing Adsorption [PSA] process[2].
2. Partial Oxidation
The synthesis of hydrogen for use in automobile fuel cells and other commercial applications has been proposed using partial oxidation (POX) and catalytic partial oxidation (CPOX) of hydrocarbons. The gasified raw material is often heavy oil fractions (such as vacuum remnants, and heating oil), which are difficult to treat further and utilize. Methane and biogas are also possible. In the noncatalytic POX process, the raw material is gasified at temperatures between 1300 and 1500°C and pressures between 3 and 8 MPa while being exposed to oxygen (Equations (5),(6)) and potentially steam (7), ATR). More CO (H2:CO = 1:1 or 2:1) is created when compared to steam reforming (H2:CO = 3:1). To complete the process, CO is reacted with steam and converted into H2 and CO2[3].
CH4 + O2 → CO + 2H2 Equation 5
CH4 + 2O2 → CO2 + 2H2O Equation 6
CH4 + H2O (g) → CO + 3H2 Equation 7
Several gases, including CO, CO2, H2O, H2, CH4, hydrogen sulphide (H2S), and carbon oxysulfide, are produced during partial oxidation (COS). A portion of the gas is burned to generate adequate heat for the endothermic reactions. As an undesired intermediate product, the soot produced by the breakdown of acetylene is produced. The quantity is determined by the H:C ratio of the original raw fuel. Therefore, like the SR, efforts have been made to switch to raw materials with a greater H: C ratio, including natural gas[3].
B. Black/Brown Hydrogen Production Methods
This generation uses coal as a fuel for burning and lies at the end of the spectrum on the colour code as this method has collateral damage to nature. In this process, the production of one ton of hydrogen results in the production of 19 tons of carbon dioxide.
- Thermal Cracking of Natural Gas
An advanced method for producing hydrogen from fossil fuels is the thermal cracking of natural gas. It is used to produce carbon black for the printing and pigment industries as well as for the vulcanisation of rubber tires. In this process, hydrogen is also produced and used to provide some of the process' heat energy. Tandem furnaces are used to carry out the process batch-wise at pressures close to atmospheric pressure. The firebrick is heated to 1400oC using a methane-air flame. Then, the air is shut off, leaving only the methane to break down on the hot firebrick as the temperature falls to about 800oC. Bag filters are used to catch the micron-sized carbon particulates in the gas stream. The second furnace is then heated using the methane-hydrogen gas as the first furnace's methane decomposition process proceeds. Then, the flow is switched around such that the second furnace is heated up while the first furnace is creating carbon black. About 40% of the entire energy output can be recovered as carbon black, and about 60% can be recovered as hydrogen[2].
2. Coal Gasification
The chemical processes of coal gasification are very similar to those of the partial oxidation of heavy oils. In coal gasification, a hydrogen-rich gas is created, which is then separated into hydrogen and other gases using a variety of procedures.[2]
The two methods for producing hydrogen from coal are the Synthane process and the CO2 acceptor process.
The gaseous products of the reaction between steam and coal at 450 pressure and 800-900? include CO, CO2, and H2. There is some methane production. When the pressure is raised to 1000 psi, methane becomes a significant product. Monoethanolamine or potassium hydroxide is used to wash the final gas to eliminate the CO2 gas. Only 97 to 98% of the original gas remains pure. The by-product is CO2[2].
The CO2 acceptor process, an alternate method, uses lime, which is added to the coal when it combines with steam. Lime absorbs the created CO2 gas as calcium carbonate. The shift reaction takes place in the main reactor once the CO2 has been removed, therefore no external shift reactor or washing is required to remove CO2. The calcium carbonate can be heated in a separate reactor to release CO2 into the environment and make lime. In fluidized beds operating at 450 psi, gasification is carried out for both the steam-oxygen and the CO2 acceptor processes[2].
C. Blue Hydrogen Production Methods
The generation uses fossil fuels as a primary source for burning and is further supported by carbon capture to prevent the emission of greenhouse gases. In this process, the production of one tonne of hydrogen results in the production of 12 tonnes of carbon dioxide.
- Autothermal Reforming
Steam is added during the catalytic partial oxidation process in auto thermal reforming (ATR). ATR combines partial oxidation (exothermic) and steam reforming (endothermic) processes. ATR is better than SR of methane since it doesn't need external heat and is easier and less expensive[3].
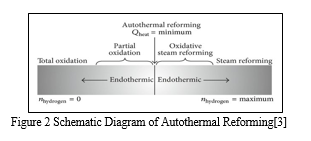
The above figure shows the fuel processor's operational range for producing hydrogen. The specific aim determines the operating conditions for the reformer. The high hydrogen yield with low carbon monoxide content is a primary goal. Steam reforming allows for high hydrogen efficiency and little carbon monoxide. However, because steam reformation is an endothermic process, it requires a lot of energy. The system needs to receive this energy from the outside[3].
ATR has another important advantage over the SR method in that it may be stopped and started extremely quickly and produces more hydrogen than POX alone. The thermal efficiency for reforming methane is like that of POX (about 60 and 75 %) and somewhat lower than that of steam reforming. Using the auto thermal process and proper catalysts, gasoline, and other higher hydrocarbons can be turned into hydrogen for use in automobiles on board[3].
D. Green Hydrogen Production Methods
Green hydrogen is produced without the release of any damaging greenhouse gases. By electrolyzing water with clean electricity generated from renewable sources of energy like solar, wind, and biomass, green hydrogen can be produced. Currently, renewable energy systems are under optimization and cost-cutting process so Green Hydrogen is expensive to be generated at an industrial scale but it will soon be normalized with the rapid growth of technology. Currently, Green Hydrogen production takes as much as 245 KJ/mole of Hydrogen produced where the yield of Hydrogen is 1 mole of H2 per mole of feed.
2. Photolysis
In theory, hydrogen can be released when water molecules absorb 285.57 kJ/mole of energy from ultraviolet radiation. To release the formed gases, some photocatalysts are utilized to absorb visible light and convey the energy of the right wavelengths and intensity of water molecules. With a photocatalyst "X," the photolysis can be expressed as follows[2]:
H2O + X + Light → Reduced X + 2H+ + 1/2O2 Equation 8
Reduced X + 2H- → X + H2 Equation 9
H2O + X + Light → H2 + 1/2O2 + X Equation 10
The photocatalysts' Xs include a variety of compound salts, compound semiconductors, photosynthetic dyes, complete cells of various blue-green, green, and red algal species, photosynthetic bacteria, and more. This approach is direct, uses ordinary light, delivers the photocatalyst back and has very low efficiency[2].
Colloidal platinum was shown to be the most effective catalyst for the reaction of hydrogen evolution, and colloidal ruthenium and/or titanium dioxide for the reaction of oxygen evolution[2].
3. Thermochemical water splitting
High-temperature heat (500°–2000°C) is used in thermochemical water splitting systems to power a series of chemical reactions that yield hydrogen. Each cycle of the process involves the usage of the same chemicals, resulting in a closed loop that uses only water to create hydrogen and oxygen. The following techniques can be used to produce fundamentally extreme temperatures: (a) Using a "heliostat" field of mirrors to focus sunlight onto a reactor tower or (b) advanced nuclear reactor waste heat. More than 300 water-splitting cycles are depicted in the writing. Two examples are (i) the "direct" two-step cerium oxide warm cycle which is less complex with fewer steps and requires higher operating temperatures and (ii) the "hybrid" copper chloride cycle.
4. Biomass Gasification
Animal waste, municipal solid waste, crop leftovers, short-rotation woody crops, agricultural waste, sawdust, aquatic plants, short-rotation herbaceous species (like switch grass), wastepaper, corn, and a variety of other materials are all sources of biomass. Gasification technology is widely utilized in various processes and is quite developed. It is frequently used with biomass and coal as fuel feedstocks. It is a type of pyrolysis that relies on the partial oxidation of the feedstock material to produce "producer gas," which is a mixture of hydrogen, methane, higher hydrocarbons, carbon monoxide, carbon dioxide, and nitrogen[3].
The moisture in the biomass must also be evaporated during the gasification process, which results in low thermal efficiency. It can be carried out in a fixed-bed or fluidized-bed reactor and with or without a catalyst, with the latter reactor often having greater performance. The gasification process produces "syngas" with an H2/CO ratio of 2/1 when steam and/or oxygen are added. These "syngas" is then fed into a Fischer-Tropsch reactor to produce higher hydrocarbons (synthetic gasoline and diesel), or a WGS reactor to produce hydrogen. High hydrogen yields have been achieved by reforming dry biomass with superheated steam (about 900°C). However, even when operating at temperatures between 800 and 1000°C, the gasification process contributes appreciable amounts of "tars" (a complex mixture of higher aromatic hydrocarbons) to the output gas. The resulting gas is catalytically cleaned and upgraded in a secondary reactor using calcined dolomite and/or nickel catalyst[3].
5. Water Electrolysis
Electrolysis of water is one of the most capable methods to produce hydrogen because the fuel used to produce hydrogen is H2O which is renewable and as a by-product produced only pure oxygen. The electrolysis process utilizes the DC power obtained from sustainable energy resources such as solar, wind, and biomass. But, at present only ?4% of hydrogen can be obtained by electrolysis of water mainly due to economic issues[4]. Water electrolysis can be classified into four types based on the electrolyte, operating conditions, and ionic agents (OH-, H+, O2-), however, operating principles are the same. The four types of electrolysis methods are as follows[4]:
Alkaline water electrolysis (AWE)- Alkaline electrolysis uses an aqueous solution (KOH/ NaOH) as the electrolyte, with a concentration of 20 –30 wt% , and operates at lower temperatures, such as 30-80°C. The asbestos diaphragm (old)/ Zirfon®(current), which is located in the middle of the cell and divides the cathode and anode as well as the created gases from their respective nickel electrodes, prevents the electrolysis process from mixing the produced gases[4].
During the initial stages of the AWE process, two molecules of the alkaline solution (KOH/NaOH) are reduced to one hydrogen (H2) molecule and two hydroxyl ions (OH-) are formed at the cathode side. The hydroxyl ions (OH-) transfer under the influence of an electrical circuit between the anode and the cathode through the porous diaphragm to the anode, where they are discharged to a half molecule of oxygen (O2) and a molecule of water (H2O). The produced H2 is eliminated from the cathode surface to recombine in a gaseous form. As a last innovation, anion exchange membranes (AEM) constructed of polymers with anionic conductivity are being developed to replace the asbestos diaphragm in alkaline electrolysis[4].

PEM water electrolysis- In PEM water electrolysis, hydrogen, and oxygen is electrochemically separated from water at the appropriate electrodes, such as the cathode for hydrogen and the anode for oxygen. Pumping water to the anode, where it splits into oxygen (O2), protons (H+), and electrons (e-), initiates PEM water electrolysis. The proton-conducting membrane transports these protons to the cathode side. The reaction's driving force (cell voltage) is provided by the electrons leaving the anode through the external power circuit. Hydrogen is produced at the cathode side by the recombination of protons and electrons[4]. A PEM fuel cell and a PEM water electrolyzer have very similar designs. The Membrane Electrode Assembly(MEA), which is located between two electrically conductive plates, is the heart of a PEM water electrolyzer. It consists of a PEM covered with catalyst-containing electrodes that serve as the anode and cathode. The membrane electrically isolates the electrodes from one another because it only conducts protons[5].
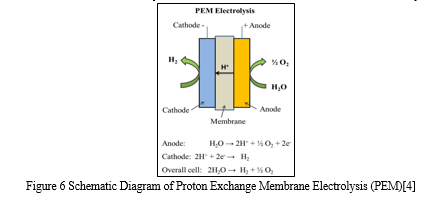
Table 1 Advantages and Disadvantages of different water electrolysis methods[4].
|
Electrolysis Process |
Advantages |
Disadvantages |
|
Alkaline Electrolysis |
Well-established technology, Non-noble electrocatalysts, Low-cost technology, the energy efficiency is (70-80%), Commercialized. |
With low current densities, Low purity of gases, Low operational pressure (3-30 bar), and Low dynamic operation, The formation of carbonates on the electrode decreases the performance of the electrolyzer. |
|
Solid Oxide Electrolysis |
Has Higher Efficiency (90-100%), Non-noble electrocatalysts, High working Pressure. |
Laboratory stage, Large system design, Low durability. |
|
Microbial Electrolysis
|
Used in different organic wastewaters. |
Under development and low purity of hydrogen, Low hydrogen production rate because of high internal resistance, electrode materials, and complicated design. |
|
PEM Electrolysis |
High current densities, Compact system design and Quick Response, Greater hydrogen production rate with High purity of gases (99.99%), Higher energy efficiency (80-90%). High dynamic operation. |
New and partially established, High cost of components, Acidic environment, Low durability, Commercialization is in the near term.
|
E. Turquoise Hydrogen Production Methods
The generation uses the Methane Pyrolysis method which is one of the predominant methods in industrial-scale Hydrogen generation. If the heating process is fueled by renewables and the carbon is either permanently stored or consumed, turquoise hydrogen may one day be recognised as low-emission hydrogen.
- Pyrolysis and Copyrolysis
In the region of 500-900°C, hydrocarbon is heated and gasified at 0.1-0.5 MPa pressure. The process occurs in an environment devoid of oxygen and air, virtually eliminating the possibility of dioxin production. There is no need for secondary reactors (WGS, PrOx, etc.) because no water or air is present, which prevents the formation of carbon oxides (e.g., CO or CO2). As a result, this method significantly reduces emissions. Although the materials haven't been cured, if air or water is present, considerable COx emissions will be generated. Fuel flexibility, relative simplicity and compactness, clean carbon byproduct, and decreased COx emissions are some benefits of this technology. The following equation serves as a generic description of the reaction[3]:
CnHm + heat → nC + 0.5 m H2 Equation 11
Pyrolysis processes are classified as low (up to 500°C), medium (500–800°C), and high (beyond 800°C) depending on the temperature range. One of the most recent methods for converting organic material into goods with a higher energy content is fast pyrolysis. The full spectrum of produced phases contains the byproducts of rapid pyrolysis (solid, liquid, and gaseous). It has the potential for lower CO and CO2 emissions and may be operated in a way that recovers a considerable proportion of solid carbon that is easily sequestered[3].
Table 2 Summary of Hydrogen Production Technologies[3], [4]
|
Technology |
Feedstock |
Advantages |
Disadvantages |
Efficiency |
Maturity |
|
Steam Reforming |
Hydrocarbons |
Developed technology & Existing infrastructure |
Produced CO, CO2 Unstable supply |
74-85 % |
Commercial |
|
Partial Oxidation |
Hydrocarbons |
Established Technology |
Along with H2 Production, produced heavy oils and petroleum coke
|
60-75 % |
Commercial |
|
Autothermal Reforming |
Hydrocarbons |
Well established t, existing infrastructure |
Produced CO2 as a byproduct, use of fossil fuels |
60-75 % |
Near Term |
|
Pyrolysis |
Hydrocarbons |
Clean and sustainable, O2-byproduct, copious feedstock
|
High capital costs, Elements toxicity, corrosion problems.
|
35-50 % |
Near Term |
|
Biomass Gasification |
Biomass |
Abundant, cheap feedstock and neutral CO2.
|
Fluctuating H2 yields because of feedstock impurities, seasonal availability and formation of tar. |
35-40 % |
Commercial |
|
Electrolysis |
H2O + electricity |
Established technology Zero emission Existing infrastructure O2 as byproduct
|
Storage and Transportation problem.
|
60-80 % |
Commercial |
|
Photolysis |
H2O + sunlight |
O2 as byproduct, abundant feedstock, No emissions.
|
Low efficiency, non-effective photocatalytic material, Requires
|
0.06 %* |
Long Term |
|
Thermochemical Water Splitting |
H2O + heat |
High efficiency, low cost, and no need for electricity |
High temperature of the cycle, the formation of an azeotropic compound |
NA |
Long Term |
*Hydrogen Purification is not included.
III. STORAGE METHODS
Compared to 12 kWh of petrol and diesel, hydrogen contains 33.33 kWh of energy per kilo. But hydrogen requires a larger volume for storing the same amount. Hydrogen as compressed gas and cryogenic liquid, underground storage for large-scale applications turns out to be a preferable method. Solid-state hydrogen storage has seen rapid development and is believed to be the safest hydrogen storage mode in recent years. Different technologies of hydrogen storage have been summarized as follows[6]:
A. Compressed Gas
Hydrogen under a pressure of up to 700 bar and compressing it into steel gas cylinders is one of the options. Under 700 bar, it reaches a volumetric density of 36 kg/m3. State-of-the-art lightweight composite steel high-pressure gas cylinders are used [5]. When transporting hydrogen through a hydrogen pipeline and hydrogen tube trailer compressed hydrogen storage is widely used, but there is a limitation in the capability of transport due to the weight of the gas cylinder[6].
- Storage vessels (Pressure Vessels to store Hydrogen)
Hydrogen adsorption and dissociation at the surfaces of some metals can produce embrittlement, which lowers the material's strength and endurance. Austenitic stainless steel, aluminium, and copper alloys are suitable materials that are frequently used for pressure cylinders because they are known for their resistance and opposition to the effects of hydrogen at ambient temperatures. The use of many other materials, such as alloy or high-strength steels, should be avoided in hydrogen storage applications since they are prone to embrittlement[7].
There are four different types of pressure vessels used to store hydrogen: Type I, II, III, and IV. The pressure vessels are typically built with a polar entrance, two spherical domes, and a center cylindrical part. When it comes to composite vessels, they could also be polymorphic or toroidal[7].
Table 3 Different Types of Vessels
|
Type |
Description |
|
Type I |
Type I pressure vessels are available with a net capacity of 2.5–50 m3 at a pressure of 200–300 bar; they are typically utilized in industrial and commercial applications. The highest pressure they can withstand is 500 bar. Built of metals such as carbon steel and low alloy steel. Have good safety and strength characteristics, but because of their heavy weight different tanks with substantially lower weights are developed[7]. |
|
Type II |
Type II features a thick steel or aluminium load-bearing metal lining that provides gas tightness and stops gas outflow. This thick liner's cylindrical portion has a fiber resin composite partly wrapped around it in a hoop-like pattern. The maximum stress that the metal can withstand before becoming brittle will be decreased as a result of this hoop reinforcement, which will increase resistance to metallic liner fatigue (tensile stress). The steel liner and the composite material each carry an equal share of the structural load. Type II vessels are capable of withstanding indefinite pressure[7]. |
|
Type III |
Type III pressure vessels are made of a metal liner that is thin and completely wrapped (axially and around the hoop) with a fiber resin composite of high stiffness and strength. This composite carries the majority of the pressure load operating on the vessel. In comparison, only roughly 20% of the load is supported by metal liners. Type III is half as heavy as type I and can sustain pressure up to 450 bar, but it costs twice as much as type II[7]. |
|
Type IV |
A Type IV vessel has a polymer liner, which ensures gas tightness, or, in rare instances, an incredibly thin metal liner that is completely encased in a fibre resin composite. The only remaining metal components are the vessel boss and its liner junction. The storage pressure allowed by these containers is up to 1000 bar. Although these vessels are the lightest of all vessels, their prices are quite high because the structural weight is carried by composite materials[7]. |
|
Type V |
A brand-new class of vessels known as type V, a linerless completely composite pressure vessel built on a fiber-reinforced shell, was unveiled in 2010 by Composites Technology Development Inc. The first vessel of this sort was 102% lighter and cheaper than storage vessels made of composite materials (Type II to IV). However, Type V is not a good fit for large-scale compressed hydrogen storage due to the high design and development costs, as well as the fact that Type V's maximum operating pressure and volume are currently constrained[7]. |
2. Underground Hydrogen Storage
For large-scale hydrogen storage in medium and long-term hydrogens underground storage solutions, such as aquifers, depleted deposits of natural gas and oil and salt caverns are the principal choices[6]. The capacity of the first two types of porous structures may be influenced by geological conditions. Approximately 75% of the most underground hydrogen storage in the world is in depleted deposits[6].
Salt Caverns- In massive subsurface salt deposits, salt caverns are primarily cylindrical, man-made holes that are constructed from the surface by carefully injecting water into a well in the salt rock. The name of this procedure is solution mining[8].
The salt caverns are a suitable choice for hydrogen storage due to the unique geological circumstances, such as tightness, the advantageous mechanical qualities of salt, and its impedance to chemical processes. Additionally, evaporitic rocks' viscoplastic qualities contribute to their improved sealing capabilities, and given that salt caverns are mechanically stable, this makes the injection-withdrawal process versatile and suited for medium- and short-term storage. High salinity level limits the ability of bacteria to consume hydrogen. Cave’s depth impact storage capacity and affects pressure which in turn affects how much-compressed gas is there[8]. A lower depth requires less cushion gas, which helps to cut the operation's cost. Gas may be injected and retrieved numerous times a year from storage facilities in salt caverns because they are simple to maintain. They are among the best options for maintaining peak gas reserves[8].
Aquifer- Aquifers are porous, permeable mediums that allow fresh or salt water to pass via their pore spaces. To store hydrogen in underground formations like deep aquifers, two requirements must be met such as the host rock must have strong reservoir characteristics and there must be an impermeable barrier to stop the gas from migrating[8].
Due to the density difference between gas and liquid, when hydrogen is pumped into an aquifer that is full of water, the liquid will go downhill or to the sides. In this instance, adding hydrogen in the same volume without removing any liquid increases the pressure of the porous media, changing the liquid-gas interface as a result during the injection procedure. A drawback of hydrogen in aquifers is that when it is going to be removed, the liquid and gas can both be created concurrently due to the movement of the gas-liquid interface. Numerous events, including leaking along hidden fractures, biological reactions, or interactions between hydrogen and minerals in the reservoir rock, can have an impact on the performance of a hydrogen storage facility[8].
Depleted Oil and Gas Reservoir- Depleted gas reservoirs are among the underground geological features that are well described and have practically all of the formation's relevant data available. Additionally, it has been demonstrated that the caprocks of depleted gas reservoirs are tight. Because it can serve as cushion gas, the presence of the leftover gas in a depleted gas reservoir is seen as advantageous. However, if the residual gas can lower the hydrogen's purity, it could be considered a drawback[8].
New Underground Gas Storage (UGS) facilities typically reach their intended exploitation parameters in five years or less. During this period, formation waters that infiltrated the gas deposit after it stopped being used are being ejected. The site's maximum pressure during UGS frequently exceeds the initial reservoir pressure. By doing this, more gas than was originally offered in the deposit can be stored. A thorough analysis is needed before turning a depleted oil reservoir into a hydrogen storage location. At the hydrogen-oil interface, several contact interactions between the two substances are conceivable, which lowers the purity of the hydrogen. Hydrogen may also be lost because of hydrogen dissolving in leftover oil[8].
B. Liquid Hydrogen
At a low temperature (20–21 K) and ambient pressure hydrogen can be converted into its liquid form. It was realized that volumetric density can reach 70.8 kg/m3, which is even a little bit higher than that of solid hydrogen, i.e. 70.6 kg/m3. About 40% of energy is lost during the liquefaction process and it is time and energy-consuming to liquefy the hydrogen. Right now, liquid hydrogen is reserved for special high-tech applications, e.g. space travel, and has not yet been largely commercialized[6].
The Joule–Thompson cycle (Linde cycle) is the simplest liquefaction cycle. The gas is first compressed and then cooled in a heat exchanger before it undergoes an isenthalpic Joule–Thomson expansion, producing some liquid when it passes through a throttle valve. The cooled gas is separated from the liquid and is returned to the compressor via the heat exchanger.At room temperature, hydrogen warms upon expansion. The temperature must be below its inversion temperature of 202 K for hydrogen to cool upon expansion. Therefore, liquid nitrogen (78 K) is usually used to pre-cooled hydrogen before the first expansion step occurs. The free enthalpy change between liquid hydrogen at 20 K and gaseous hydrogen at 300 K is 11,640 kJ·kg-1. The necessary theoretical energy (work) to liquefy hydrogen from RT is Wth=3.23 kWh·kg-1, and the technical work is about 15.2 kWh·kg-1, almost half of the lower heating value of hydrogen combustion. Since it has the lowest surface-to-volume ratio and has a uniform distribution of stress and strain, a sphere is an ideal shape theoretically[9].
C. Solid Storage
Solid hydrogen is realized by combining hydrogen with solid materials through absorption and adsorption. To formulate chemical compounds absorption stores the hydrogen directly into the bulk of the material. Among them, metal hydrides have aroused more interest owing because of their high hydrogen storage capacity. At room temperature and atmospheric pressure Palladium can absorb 900 times its volume of hydrogen[6]. Lowering the cost, optimizing the operation temperature, and enhancing the thermal management of the system are the efforts made for large-scale development. Some complex hydrides (Mg2NiH4, LiAlH4, NaBH4, etc.) and chemical hydrides (LiH, NaH, CaH2, etc.) store the hydrogen by absorption, but the main challenges of these methods are the lack of reversibility and the complex reactions to extract hydrogen[6]. Another choice for storing hydrogen is by adsorption in which porous materials, such as metal-organic frameworks and carbon materials physically adsorb hydrogen[10]. Thermal management in the charging and discharging process can be avoided. The physical adsorption of hydrogen storage is still far away from large commercialization because the filling time is still under satisfaction when considering the storage capacity[6].
Another class of lightweight storage materials is complex hydrides. Complex hydrides are known as “one-pass” hydrogen-storage systems which means that H2 evolves upon contact with water. Sodium, lithium and beryllium are the only elements lighter than magnesium that can form solid-state compounds with hydrogen. The hydrogen content reaches the value of 18 wt% for LiBH4. The use of complex hydrides for hydrogen storage is challenging because of both kinetic and thermodynamic limitations[11].
IV. APPLICATION IN POWER SYSTEMS
The four typical applications of integrating hydrogen into power systems are energy storage, power to gas, fuel cell co-generation, and tri-generation and vehicular applications[6].
A. Energy Storage
It can satisfy energy storage needs in a large time-scale range varying from short-term system frequency control to medium and long-term (seasonal) energy supply and demand balance[6].
- Medium to long-term energy storage
Renewable energy generation in recent years has seen rapid growth. The intermittent nature of some renewable energy resources makes their time and season dependent. The generated renewable energy needs to be stored in a reliable form, which should be tolerant to the fluctuation and randomness of those renewable energy sources [6]. Pumped hydro energy storage, compressed air energy storage, batteries, etc. are some of several existing energy storage options. Hydrogen has its advantages because of its high energy storage capacity, long storage period, and flexibility. It can smooth out energy volatility and uncertainty and absorb the excess renewable energy generation. It can be applied to deal with energy time shifts and seasonal variation[6]:
Energy time shift: When the supply is larger than the demand and when it is needed hydrogen is used to equilibrate the demand and supply by storing the excess energy generated by renewables and the hydrogen can be used for power generation or grid injection through stationary fuel cells. Energy generated during low demand and low electricity price periods tends to be stored in hydrogen to lower the energy cost and on the contrary for gaining the most benefit, the hydrogen is used to produce electricity during high demand and high electricity price periods. The storage duration is much longer than batteries, up to weeks or months, compared to hourly or weekly storage of batteries[6].
Seasonal variation: Renewable resources can be shifted across the seasons due to the seasonal difference in energy production through hydrogen. Due to its high energy density, hydrogen storage capacity can reach up to MWh, even TWh, while one needs to expand the size of the instrument to reach greater storage capacity because batteries tend to be used in kWh to MWh applications[6].
B. Power-to-Gas
Power-to-gas is an application that produces combustible gas using electric power. With rather high energy density hydrogen is believed to be a combustible gas, and power-to-hydrogen applications are gaining momentum. Providing a balancing service to the energy market, the hydrogen produced by an electrolyzer can then be methanated into methane and injected into the natural gas grid, or stored [5]. Hydrogen produced by Power-to-gas can be injected into the existing gas grid. Using existing infrastructure and saving construction costs it offers an excellent storage option. With or without the methanation process, most power-to-gas projects today, tend to be pilot projects that last for 1 to 3 years, while large industrial plants are planned around the world and need more social and political support [5]. The potential improvements lie in the aspects of hydrogen and methanation producing efficiency and utilization of the by-products, like oxygen and heat[6].
C. Co-generation
Co-generation is the process of using fuel cells as prime movers to produce both electricity and heat concurrently, in which to provide the electrical needs electricity is used, while for heating applications the released heat is used so that the total efficiency can reach up to 95%. A typical fuel cell co-generation system is made up of a stack, a fuel processor (a reformer or an electrolyzer), power electronics, heat recovery systems, thermal energy storage systems (typically a hot water storage system), electrochemical energy storage systems (accumulators or supercapacitors), control equipment and additional equipment (fans, pumps, communication devices, etc.)[6].
Nowadays, a great number of commercial projects are launched to develop fuel cell co-generation applications. As homes are supplied with liquefied petroleum gas (LPG), a reformer is used to convert the LPG into hydrogen, and to heat, up the water, the residual heat can be used. Hydrogen with ambient oxygen is combined by PEMFC stack into the water and at the same time, produces electricity and heat to meet the electrical needs and to heat water, for the kitchen, bathroom, room heating, etc[6].
D. Tri-generation
Tri-generation is an extended application of co-generation, in which a prime mover to thermally driven equipment is coupled to produce cooling. To produce cold from a thermal sink usually heat pumps are used, which contain two reactors, a condenser, and an evaporator.
The two reactors consist of an absorption/adsorption reactor and a desorption reactor. In the condenser the vapour or gas extracted from the absorbent passes through it where it transforms into a liquid by rejecting heat, then at the evaporator, the refrigerant liquid passes through at low pressure, where it absorbs heat to evaporate[6].
Fuel cell tri-generation can lower carbon emissions and increase energy efficiency. In isolated applications, making full use of the fuel cell rejected heat can reduce electrical power consumption by the compressor and allows for storing cold when there is no cold demand.
The autonomy and efficiency of the system improve. A combined organic Rankine cycle (ORC) and vapour compression cooling (VCC) have been used to produce hot water and cold effect, where the fuel cell provides 8 kW electrical power. The released heat can be used to run the ORC and/or to be stored for domestic hot water supply. ORC produces mechanical power for the compressor of the VCC. Thermal solar panels are used in addition to power the ORC. They can recover 70 kW of heat and 16 kW of cold, while the overall efficiency can reach 85%[6].
E. Application of Hydrogen in Transportation.
Battery-powered vehicles suffer from range limitations while hydrogen-fuelled electric powertrains provide a solution for long-distance driving with clean energy. By 2030, 3% of global vehicle sales are expected to be hydrogen-fuelled, and this percentage could reach 36% in 2050.
To accelerate their commercialization in the vehicle market several companies are developing fuel cell powertrains in terms of their quality, reliability, and dependableness [5]. For example, Mirai fuel cell vehicles developed by Toyota have used mass-production PEMFCs with a 3.1 km/L volume power density and a 144 kW (155 DIN hp) maximum power output, where a 1.6 kWh nickel-metal hydride battery is connected in parallel to deal with the regenerative braking and also assist accelerating during high-power demands. High-pressure compressed hydrogen fuel tanks are the current hydrogen storage systems in most commercial hydrogen fuel cell vehicles. For example, Honda’s Clarity fuel cell vehicle, and Hyundai’s NEXO fuel cell vehicle use such tanks, while BMW’s Hydrogen 7 has used a liquid hydrogen fuel tank[6].
V. THE INDIAN SCENARIO
A. History of Hydrogen Production In India
Hydrogen has historically been used in India, primarily as an industrial feedstock in the production of ammonia-based fertilizers. Currently in India, most hydrogen is produced using natural gas for use in the refinery and fertilizer industries. Historically, a greater proportion of hydrogen was produced using naphtha, but this has declined primarily with the shift to of natural gas in the fertilizer sector. Coal gasification to produce hydrogen has also been used in the past but it did not pick up. Hydrogen derived from both fossil fuels and electricity has been used for many years in India, with the country’s first large-scale alkaline electrolyzer being deployed at Nangal in Punjab in 1962. It was later closed down as a result of increasing demands for electricity elsewhere in the economy and replaced by hydrogen production from natural gas[12].
B. Initiatives by Government
- National Hydrogen Energy Board
To accelerate the development and utilization of hydrogen energy in the country, the Ministry of New and Renewable Energy established the National Hydrogen Energy Board, including high-level representation from the government, industry, research institutions, and academia. In its first meeting on February 23, 2004, the National Hydrogen Energy Board decided to form a Steering Group led by Mr Ratan Tata, a Member of the Board and Chairman of Tata Sons, to develop a National Hydrogen Energy Road Map. Mr Anand Mahindra served as Co-Chairman of this Steering Group, which included representatives from government, industry, academia, and experts. The National Hydrogen Energy Road Map is an industry-driven planning process that provides long-term energy solutions to India's growing energy needs while ensuring the country's energy security[13].
2. Hydrogen Road Map
The Ministry of New and Renewable Energy (MNRE) developed a Hydrogen and Fuel Cell Road Map, published in 2006 (MNRE, 2006). In 2016, MNRE published a further report, which laid out more up-to-date plans for the Government’s ambitions for hydrogen (MNRE, 2016). This report lays out a comprehensive plan for increasing R&D activity in India across several program areas, with the 2016 report showing ambitious timelines for research activities out to 2022. Given the nascent stage of most hydrogen-based technologies, MNRE’s activities have mainly focused on increased research activity, as opposed to wide-scale deployment. Most recently, the Government of India is in the process of preparing a Hydrogen Mission, with support from the MNRE and NITI Aayog, which is expected to be unveiled in 2021[12].
Green Initiatives for Future Transportation (GIFT) and Green Initiative for Power Generation (GIPG) are two major initiatives identified in the Road Map (GIP). Through various stages of development, the Green Initiative for Future Transport (GIFT) aims to develop and demonstrate hydrogen-powered IC engines and fuel cell-based vehicles ranging from small two/three wheelers to heavy vehicles. It is acknowledged that the performance of hydrogen-powered vehicles must be comparable to commercially available consumer options in terms of performance, safety, convenience, and cost. Through various stages of technology development and demonstration, the Green Initiative for Power Generation (GIP) intends to develop and demonstrate hydrogen-powered IC engine/turbine and fuel cell-based decentralized power generating systems ranging from small watt capacity to MW size systems[14]. This initiative would aid the country in providing clean energy to rural and remote areas while also generating power for urban areas. The roadmap envisions the establishment of a decentralized hydrogen-based power generation capacity of approximately 1,000 MW aggregate capacity in the country by 2020. The Government of India has also announced incentives worth 17000 crores to promote manufacturing of electrolyzers and green hydrogen in the country.
???????3. National Hydrogen Mission
On the 75th anniversary of India's independence, the National Hydrogen Mission was introduced. The Mission aspires to make India a green hydrogen hub and assist the government in achieving its climate goals. By 2030, 5 million tonnes of green hydrogen would be produced, and the accompanying expansion of renewable energy capacity will be made possible thanks to this mission. The policy has made it easier for manufacturers to set up Green Hydrogen manufacturing plants including benefits like exemption from inter-state transmission charges for 25 years and several other benefits like open access to renewable energy generators as an incentive. The minister of finance declared a total budget for the mission of Rs. 19,700 crores ($2.3 billion). The government allocated Rs. 297 crores for the first year of the seven-year mission, 2023-24, the first-ever such allocation for increasing green hydrogen production in the country.
The mission has also laid out a year-by-year roadmap from 2022-23 to 2029-30, with work divided into two phases. There are plans for Phase I (2022-23 to 2025-26) to develop the standards, generate initial demand, conduct pilot projects, and issue a series of incentives to increase local production. Following that, in Phase II (2026-27 to 2029-30), the proposals are to expand the commercialization of green hydrogen to other sectors such as shipping, transportation, and others. This plan is based on the expectation that green hydrogen production costs will become cost-effective by 2025-26.
Following the development of such policies a recent report by OMI Foundation from February 2023, states that four Indian states, Uttar Pradesh, Rajasthan, Odisha, Tamil Nadu, and Kerala, have a policy/incentive for green hydrogen. Gujarat is planning to launch a similar initiative soon. Incentives for green hydrogen have been included in renewable energy policies in states such as Maharashtra and Madhya Pradesh.
The current Minister of Power and New and Renewable Energy stated that the country aims to acquire approximately 8 million tonnes of green hydrogen capacity by 2030. This would necessitate nearly 200 GW of renewable energy capacity. Meeting this is seen as a challenge. The cost of installing solar in India is the lowest in the world, at $600,000 per MW. After completing a survey, the government plans to auction contracts for offshore wind installation off the coasts of Gujarat and Tamil Nadu.
C. Initiatives by Industry
An independent advocacy group for green hydrogen has been established by six renewable energy companies: Azure Power, Acme Group, Fortum India, O2 Power, Sprng Energy, and SunEdison Infrastructure. The Independent Green Hydrogen Association (IGHPA) aspires to collaborate with the government and other stakeholders to help India reach its goal of becoming a hub for exporting green hydrogen and ammonia. IGHPA will contribute technical, financial, and regulatory information for the creation of the policy framework and its execution.
Some other private company initiatives are listed below:
Table 4 Industry Initiatives
|
Company |
Initiative |
|
Reliance Industries Ltd |
RIL has announced a plan to invest Rs 75,000 crore over the next three years to build a new clean-energy business. The plan is divided into three parts: a Rs 60,000-crore investment in four giga factories that will manufacture and fully integrate all of the business's critical components; a Rs 15,000-crore investment in building the value chain, partnerships, and future technologies, including upstream and downstream industries; and repurposing the company's engineering, project management, and construction capabilities towards clean energy[15]. |
|
Indian Oil Corporation Ltd |
At its refinery in Mathura, the company intends to construct the first green hydrogen plant in the nation. Additionally, the oil marketing business has received preliminary approval for an investment of Rs 100 crore to establish green hydrogen fuel infrastructure as a test site in Kochi and Thiruvananthapuram. It also plans to set up a 2000 crore hydrogen power plant at its Panipat Oil Refinery by 2025. The plant is targeted to have an annual capacity of 7000 tonnes. |
|
NTPC Ltd |
At its Simhadri plant in Andhra Pradesh, the state-run electric company won the first green hydrogen microgrid project in the nation. The company's subsidiary, NTPC Renewable Energy Ltd, announced a domestic tender in July 2021 to build Leh, Ladakh's first green hydrogen refuelling station. The business intends to produce green hydrogen on a large scale using the power produced by its planned renewable energy projects[15]. |
|
Larsen & Toubro Ltd |
To establish a facility in India, L&T agreed with HydrogenPro AS, a manufacturer of electrolyzer technology located in Norway. According to the memorandum of agreement, L&T and HydrogenPro will endeavour to establish an alkaline water electrolyzer manufacturing facility on the scale of a gigawatt using HydrogenPro technology. L&T and ReNew Power, partnered in December to jointly develop, own, carry out, and run green hydrogen projects in India[15]. |
|
Adani Group |
Adani Group plans to invest $20 billion in generating renewable energy over the next decade, with the goal of producing green hydrogen. |
|
Bharat Petroleum Corporation Ltd |
The company has collaborated with Bhabha Atomic Research Centre (BARC) to work on alkaline electrolyzer technology for Green Hydrogen production. The company aims to scale up production of the electrolyzer for commercial use, especially in refineries. By 2030, Indian public sector oil refineries plan to build 137 kilotonnes per year (ktpa) of green hydrogen facilities. Indian Oil plans to first build a 7 ktpa electrolysis plant at its Panipat refinery[15]. |
|
ACME Group |
ACME Group, an Indian renewable energy developer, signed a memorandum of understanding (MoU) with the Karnataka government in June 2022 to develop an Rs52,000 crore (US$7 billion) integrated solar-to-green hydrogen-to-green ammonia facility. In Karnataka, the facility will produce 1.2 million tonnes per year (mtpa) of green hydrogen by 2027. To support the project's execution, the state government may facilitate project land, off-takers, and export-related facilities. ACME Solar, a subsidiary of the ACME Group, has completed the world's first commercial pilot of a green hydrogen and green ammonia manufacturing plant in Bikaner, Rajasthan. |
|
ReNew Power |
Another Indian renewables developer looking to combine its knowledge and experience in the green hydrogen space is ReNew Power. In April 2022, it announced a green hydrogen production joint venture with the state-run Indian Oil Corporation (IOC) and construction and engineering behemoth Larsen & Toubro (L&T). |
D. Fuel Cell Research & Development
A comprehensive Research, Development, and Demonstration (R&D) program on hydrogen energy and fuel have been supported by the Ministry of New and Renewable Energy (MNRE). The production of hydrogen from renewable energy sources, its safe and effective storage, and its utilization for energy and transportation purposes through combustion or fuel cells are challenges that are being addressed in industrial, academic, and research institutions. The major transportation-related study has been funded by Mahindra & Mahindra, IIT Delhi, and Banaras Hindu University. As a result, internal combustion engines, two- and three-wheeled vehicles, and hydrogen-fueled minibuses have been developed and put on display. There are now two hydrogen refueling stations at the Indian Oil R&D Centre, Faridabad and the National Institute of Solar Energy, Gurugram[16].
The Union Ministry for Road Transport and Highways March 2022 launched a pioneering hydrogen-based advanced Fuel Cell Electric Vehicle (FCEV) pilot project that will mark a significant move away from fossil fuels and toward the preservation of our environment. This pilot project was started by Toyota Kirloskar Motor Pvt. Ltd. and the International Center for Automotive Technology (ICAT) to research and assess the world's most advanced FCEV Toyota Mirai, which runs on hydrogen, in India. The Toyota Mirai is propelled by a hydrogen fuel cell battery pack and has a range of up to 650 kilometers on a single charge with a five-minute refueling period[17].
The Ministry of Ports, Shipping, and Waterways chose Cochin Shipyard Ltd. to design and construct India's first indigenous hydrogen-fueled electric vessels (CSL). Fuel Cell Electric Vessel (FCEV), a hydrogen fuel cell vessel based on Low-Temperature Proton Exchange Membrane Technology (LT-PEM), is anticipated to cost approximately Rs. 17.50 crores, of which 75% will be covered by the centre[18].
According to International Partnership for Hydrogen and Fuel Cells in the 'Economy', India currently has 58 hydrogen fuel cell buses and 2 refueling stations.
Conclusion
Electrolysis requires around 9 litres of fresh water to produce one kg of hydrogen (and 8 kg of oxygen). India’s hydrogen demand with electrolysis would require around 54 million cubic meters today, rising to approximately 270 million cubic meters by 2050. India’s total usable water supply is between 700 billion cubic meters and 1,200 billion cubic meters, although this is declining with overuse and climate change (NBR, 2013). Based on this, water requirements for electrolysis would consume around 0.05% of India’s water supply. [10] Hydrogen is produced annually through various sources, including natural gas, coal, fossil fuels, oil, and by-products in other industrial processes. India produces 6 million Metric Tons of hydrogen annually, with 2.6 million Metric Tons used in refineries, 3.2 million Metric Tons in the Chemical and Fertilizer industries, and the remaining for other purposes. Hydrogen is considered an alternative fuel for automobiles due to its ability to power fuel cells in zero-emission vehicles, domestic production, and high fuel cell efficiency. The new hydrogen economy is a sustainable energy supply, with renewable energy being the most attractive. However, hydrogen\'s primary use remains in the production of ammonia, metals, and electronics, with an annual global consumption of around 40 million tons. Large-scale hydrogen generation is needed to meet this large-scale hydrogen consumption. Methods of hydrogen production include steam methane reforming (SMR), partial oxidation (POX) which are grey production methods. Steam reforming is a popular and affordable process, while partial oxidation (POX) and catalytic partial oxidation (CPOX) of hydrocarbons are proposed for hydrogen synthesis. Black/Brown production method include Thermal cracking of natural gas and coal gasification. Coal gasification is another method for producing hydrogen from coal, with the Synthane process and the CO2 acceptor process being the two methods. Blue production methods include Auto-thermal reforming (ATR). Green hydrogen production methods use clean electricity from renewable sources and can use following methods photolysis, thermochemical water splitting, biomass gasification, water electrolysis, and turquoise hydrogen production methods include Pyrolysis/Copyrolysis. Storage methods for hydrogen include storing in form compressed gas, liquid form and solid form. For compressed gas we can use pressure vessels such as Type I, II, III, IV and V or underground hydrogen storage solutions such as salt caverns, aquifers and depleted oil and gas reservoirs. The Joule–Thompson cycle (Linde cycle) is used to liquify hydrogen. Metal hydrides are used to store in solid state. The four typical applications of integrating hydrogen into power systems are energy storage, power to gas, fuel cell co-generation, and tri-generation and vehicular applications. Hydrogen-fueled electric powertrains offer clean energy for long-distance driving, with a potential 36% of global vehicle sales by 2050. Companies are developing high-quality, reliable fuel cell powertrains, such as Toyota\'s Mirai fuel cell vehicles, which use high-pressure compressed hydrogen fuel tanks as their current storage systems. The European Investment Bank, a bank of the European Union, has agreed with India Hydrogen Alliance (I2HA) to provide one billion euros to develop large-scale green hydrogen centers and developments across India. Looking at the current scenario of projects implemented by both the private and the public sector on large scale green hydrogen production and development and deployment of hydrogen vehicles, we can say that India is moving to a greener hydrogen economy.
References
[1] “Alternative Fuels Data Center: Key Federal Legislation.” https://afdc.energy.gov/laws/key_legislation (accessed Jun. 18, 2023). [2] R. Kothari, D. Buddhi, and R. L. Sawhney, “Sources and technology for hydrogen production: A review,” Int. J. Glob. Energy Issues, vol. 21, Feb. 2004, doi: 10.1504/IJGEI.2004.004707. [3] C. Kalamaras and A. Efstathiou, “Hydrogen Production Technologies: Current State and Future Developments,” Conf. Pap. Energy, vol. 2013, pp. 1–9, Jan. 2013, doi: 10.1155/2013/690627. [4] S. S. Kumar and V. Himabindu, “Hydrogen production by PEM water electrolysis – A review,” Mater. Sci. Energy Technol., vol. 2, no. 3, pp. 442–454, 2019, doi: https://doi.org/10.1016/j.mset.2019.03.002. [5] T. Smolinka, E. Ojong, and J. Garche, “Hydrogen Production from Renewable Energies—Electrolyzer Technologies,” in Electrochemical Energy Storage for Renewable Sources and Grid Balancing, 2015, pp. 103–128. doi: 10.1016/B978-0-444-62616-5.00008-5. [6] M. Yue, H. Lambert, E. Pahon, R. Roche, S. Jemei, and D. Hissel, “Hydrogen energy systems: A critical review of technologies, applications, trends and challenges,” Renew. Sustain. Energy Rev., vol. 146, p. 111180, 2021, doi: https://doi.org/10.1016/j.rser.2021.111180. [7] A. M. Elberry, J. Thakur, A. Santasalo-Aarnio, and M. Larmi, “Large-scale compressed hydrogen storage as part of renewable electricity storage systems,” Int. J. Hydrog. Energy, vol. 46, no. 29, pp. 15671–15690, 2021, doi: https://doi.org/10.1016/j.ijhydene.2021.02.080. [8] D. Zivar, S. Kumar, and J. Foroozesh, “Underground hydrogen storage: A comprehensive review,” Int. J. Hydrog. Energy, vol. 46, no. 45, pp. 23436–23462, 2021, doi: https://doi.org/10.1016/j.ijhydene.2020.08.138. [9] A. Züttel, “Hydrogen storage methods,” Naturwissenschaften, vol. 91, pp. 157–72, Apr. 2004, doi: 10.1007/s00114-004-0516-x. [10] “HYDROGEN – Dr Rajiv Desai.” https://drrajivdesaimd.com/2022/03/04/hydrogen/ (accessed Jun. 18, 2023). [11] B. Sakintuna, F. Lamari-Darkrim, and M. Hirscher, “Metal hydride materials for solid hydrogen storage: A review,” Int. J. Hydrog. Energy, vol. 32, no. 9, pp. 1121–1140, 2007, doi: https://doi.org/10.1016/j.ijhydene.2006.11.022. [12] W. Hall, T. Spencer, G. Renjith, and S. Dayal, “The Potential Role of Hydrogen in India”. [13] “Ministry of New & Renewable Energy - Government of India.” https://mnre.gov.in/ (accessed Jun. 21, 2023). [14] I. F. J. Selvaraj, “Hydrogen Buses: An Analysis with a Focus on India’s Hydrogen Roadmap”. [15] “As India awaits policy, these companies already have their green hydrogen plans in place.” https://www.moneycontrol.com/news/business/as-india-awaits-policy-these-companies-already-have-their-green-hydrogen-plans-in-place-8122201.html (accessed Jun. 18, 2023). [16] “India’s Green Energy Potential | IBEF,” India Brand Equity Foundation. https://www.ibef.org/blogs/india-s-green-energy-potential (accessed Jun. 18, 2023) [17] “Hydrogen Car: All you need to know about India’s first hydrogen-powered fuel cell electric car project - The Economic Times.” https://economictimes.indiatimes.com/industry/renewables/all-you-need-to-know-about-indias-first-hydrogen-powered-fuel-cell-electric-car-project/articleshow/90284123.cms (accessed Jun. 21, 2023). [18] “Cochin Shipyard to Build Country’s First Indigenous Hydrogen-Powered Electric Vessel.” https://krishijagran.com/news/cochin-shipyard-to-build-countrys-first-indigenous-hydrogen-powered-electric-vessel/ (accessed Jun. 21, 2023).
Copyright
Copyright © 2023 Jayant Jain, Aahil Khambhawala , Rashmi Kumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54583
Publish Date : 2023-07-02
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

